Table of contents
Increasingly, product developers and manufacturers are emphasizing the importance of different forms of copper, giving preference to the copper CuI, also known as Cu+, oxidation state. There are several reasons for this, so let’s examine what’s happening behind the scenes!
Cu+ is Chuck Norris of copper?
Among the multitude of copper products, the Cu+ reduced form has been introduced as unique and special, attributed with higher absorption and utilization characteristics. The distributors of these products uniformly present the Cu+ form as superior to the Cu2+ form, often deeming the latter as disadvantageous and sometimes even dangerous.
The founder of one copper product company uses a peculiar nomenclature to view different forms of copper. They call the Cu2+ form “plant copper,” while the Cu+ form is termed “human copper.” The product pages often describe their items with the term “bioavailable,” implying physiological accessibility/utilization, and deny this property to the oxidized sibling, the Cu2+ form.
Let’s see what science has to say!
Copper is a divalent, redox-active metal, meaning it has two primary oxidation states that change according to physiological needs:
Cu(I) / Cu+ / cuprous ion
&
Cu(II) / Cu2+ / cupric ion
Thus, copper in the body can easily gain or lose an electron, thereby operating at different oxidation levels. This property makes it indispensable in energy balance and physiological processes.
The “intelligent copper” knows how to get into the cells
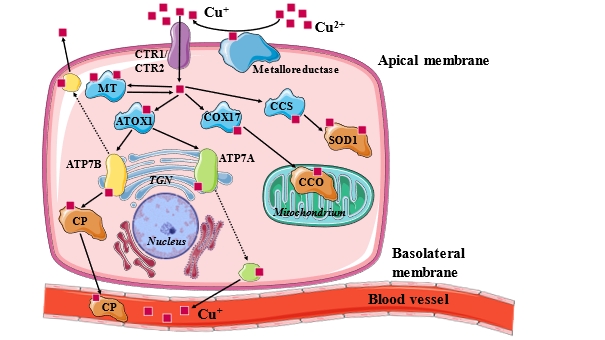
Accordingly, copper can enter the circulation through two receptors on the enterocytes of the small intestine:
CTR1 (copper transporter 1) specifically absorbs Cu1+ ions, while DMT1 (divalent metal transporter 1) can absorb Cu2+ as well as other metals in the 2+ oxidation state, including iron as Fe2+ (ferric ion). The two transporters complement each other’s function; when one is less active, the other operates with higher expression and activity. Yes, because it’s that important for copper to reach the right place.
The intimate relationship between the two transporters remains a subject of research, but numerous other mechanisms of action have been scrutinized. For example, it has been suggested that under certain circumstances DMT1 can also absorb Cu+ ions. There has also been discussion that the activity of cell surface transports is not only a function of the body’s copper needs but can also be influenced by the presence of other metals (such as those involved in iron metabolism… but more on that another time).
I’ve written more about the journey of copper in this article:
The Basics of Copper Supplementation.
Copper forges its path
If this dynamic intracellular entry route wasn’t sufficient, the special electrochemistry of copper allows it to freely oxidize or reduce between its two states, which is precisely why it is a divalent metal. Moreover, the body has its own enzymes that perform the reduction from Cu2+ to Cu+, such as STEAP proteins and Dcytb (duodenal cytochrome b).
In case of emergency, Vitamin C
Furthermore, the process is facilitated by ascorbates in the digestive system, which, through their well-known redox properties, easily induce reduction, thus converting ingested Cu2+ ions into Cu+ ions, then serving them up, so to speak, for the CTR1 transporter. Therefore, if someone were suffering from reductase deficiency due to severe genetic or toxicological effects, their body would produce alternative reductive enzymes, and they could also simply supplement their copper intake with vitamin C.
Is Cu2+ Really to Blame?
The mechanisms described above did not evolve by chance, as a large percentage of copper ingested through diet appears in the digestive system in the form of Cu2+. This is why we have enzymes that facilitate this process, and over the course of evolution, vitamin C has become our constant ally in this regard.
Furthermore, most available scientific literature has actually been conducted with Cu2+ forms, the effects of which on copper metabolism are indisputable. Among Cu2+ forms, copper sulfate stands out significantly, with the majority of studies conducted using it, thereby surpassing other forms in terms of efficacy and safety, for both Cu+ and Cu2+ forms.
It’s no coincidence that our team’s Cu-Re Drops supplement is made with pharmaceutical-grade copper sulfate.
In short, in more simple terms
- Both forms of copper have their transporters.
- We ingest both forms through our diet.
- We have numerous enzymes for reduction.
- Everyday vitamin C supplementation also facilitates copper “accessibility.”
- Cu2+ is the form more extensively studied in scientific research.
Cognitive Dissonance – So what’s behind all the hype?
To be completely honest, I’m not sure. The distributors’ pages do not provide scientific literature that substantiates their claims, and those that do are often misleading or cite studies that do not focus on copper forms.
The usual suspects can also be found here, such as those constantly cited by proponents of copper toxicity theories (e.g., Brewer GJ), focusing on topics like the copper content in plumbing, overinterpreted animal studies, and theories that have been debunked. Of course, the old fallback positions are also present, such as the role of copper homeostasis in neurodegenerative diseases, conflated with trans fats, not based on copper intake, or merely inferred from epidemiological observations. It’s as if there was a systematic avoidance of sharing any relevant studies on copper supplementation from any perspective that would demonstrate the difference between the two oxidation states (Cu1+ vs. Cu2+). Perhaps there simply aren’t any?
After a thorough analysis of the sources (and the sources cited within those sources), it still doesn’t add up for me as to why Cu+ would be superior to Cu2+. If anyone figures out what the writer intended, they get a free copper from me… the cheaper kind!

Of course, if someone really wants to, they can force their own fears or truths into the narrative, but this will not change the functioning mechanisms cited above that keep copper homeostasis in check.
So what can this Cu+ actually do?
Indeed, there are scientific studies on certain Cu+ forms (like copper(I) nicotinate), primarily in therapeutic contexts, such as in the treatment of certain types of cancer, during renal tissue toxicity, or examining its role as an antioxidant. However:
- The distributor sites did not even mention these studies.
- The studies underscore the beneficial effects of copper in general and do not make distinctions between the different forms.
The mechanisms posited above and the references cited below provide a more comprehensive framework for understanding the process. The million-dollar question, of course, is whether the proponents of Cu+ were aware of all this?
Is it possible that they wanted to create a better product and misunderstood the scientific background of copper homeostasis? Or were they driven by pure profit motive, trying to create a premium effect by marketing something as new and revolutionary? I always secretly hope for the former.
In Summary
Based on the numbers and the scientific misrepresentation, it does not seem to be a matter of mere good-willed product innovation. On the one hand, there is an abundance of scientific literature that demonstrates the efficacy of Cu2+, and specifically copper sulfate. The fact that the product developers could overlook this during product development suggests some form of either deliberate ignorance or profit motive.
However, it’s unnecessary to dissect the motivations of the product developers; these are more questions for philosophers.
In summary, purchasing and supplementing with Cu+ forms seems unnecessary and costly. The body is quite capable of managing these processes on its own, which, after all, are not complicated enzyme cascades but a simple redox process for which copper is made and why it is so important in the body.
Recommended Article: Why should we supplement with Copper?
Referencia:
- Ragaa H M Salama et al., 2007
- M el- Saadani et al., 1988
- Ahmed Medhat Hegazy et al., 2020
- Muhammad A M El-Saadani 2004
- Jesse Bertinato et al., 2004
- Joseph R Prohaska 2009
- Byung-Eun Kim et al., 2008
- Mitchell D Knutson 2007
- Martin Knöpfel et al., 2005
- https://globalhealing.com/
- mitosynergy.com